Methamphetamine exposure and chronic illness in police officers: Significant Improvement with Sauna-Based Detoxification Therapy
EXECUTIVE SUMMARY
Abstract
Background: The medical literature reports health hazards for law enforcement personnel from repeated exposure to methamphetamine and related chemical compounds. Most effects appear transitory, but some Utah police officers with employment-related methamphetamine exposures developed chronic symptoms, some leading to disability. This report is of an uncontrolled retrospective medical chart evaluation of symptomatic officers treated with a sauna detoxification protocol designed to reduce the chronic symptoms and improve the quality of life. Methods: Sixty-nine officers consecutively entering the Utah Meth Cops Project were assessed before and after a treatment program involving gradual exercise, comprehensive nutritional support and physical sauna therapy. Evaluations included pre- and post-treatment scores of the Research and Development Corporation (RAND) 36-item Short Form Health Survey (SF-36) in comparison with RAND population norms, pre- and post-treatment symptom score intensities, neurotoxicity scores, Mini-Mental Status Examination, presenting symptom frequencies and a structured evaluation of treatment program safety. Results: Statistically significant health improvements were seen in the SF-36 evaluations, symptom scores and neurotoxicity scores. The detoxification protocol was well tolerated, with a 92.8% completion rate. Conclusions: This investigation strongly suggests that utilizing sauna and nutritional therapy may alleviate chronic symptoms appearing after chemical exposures associated with methamphetamine-related law enforcement activities. This report also has relevance to addressing the apparent ill effects of other complex chemical exposures. In view of the positive clinical outcomes in this group, broader investigation of this sauna-based treatment regimen appears warranted.
Introduction
Persons addicted to methamphetamine develop serious health problems, but there is less understanding surrounding the high numbers of law enforcement personnel who experience significant symptoms associated with clandestine methamphetamine drug lab investigations (CDC, 2005). While symptoms may be transitory, many individuals have persistent symptoms causing them to seek medical attention.
Responding to an active laboratory has been associated with a 7- to 15-time higher risk of becoming ill when compared to other activities with apparently lower chemical exposures. According to Marshall (2000), since 1993 “the number of clandestine drug laboratory investigations has continued to increase, making Utah the number one state for per capita methamphetamine laboratories.”
In 2007, the Utah Attorney General investigated a sauna-based detoxification regimen operating in Manhattan for treating chronically ill rescue and recovery workers exposed during the September 11, 2001 World Trade Center attack and collapse. A senior police officer and a professional firefighter who were ill after methamphetamine lab-related exposures in Utah attributed substantial health improvement after receiving this treatment.
The nonprofit American Detoxification Foundation (ADF) established and administered the Utah Meth Cops Project (UMCP), which uses the Hubbard detoxification protocol and monitors health and quality of life among Utah police officers, to address the symptoms consistent with (and appearing after) line-of-duty exposures to methamphetamine and related chemicals.
METHODOLOGY:
Description of the Study Group, Inclusion, and Exclusion Criteria
This is a retrospective medical chart evaluation on the first 69 police officers sequentially entering the UMCP between October 2007 and July 2010. Officers were recruited through outreach efforts by project staff, word of mouth within the police community and referrals by their Chiefs of Police or County Sheriffs.
Exclusion criteria: Pregnancy, known active cancer, being wheelchair-bound, a history of psychosis, extensive psychiatric treatment, or suicide attempts were the exclusion criteria.
Inclusion criteria: (1) Law enforcement work within Utah, (2) documented contact with methamphetamine and related chemicals through law enforcement activities, and (3) the subsequent development of persistent medical symptoms or chronic ill health were the inclusion criteria. Officers gave written informed consent for treatment and outcomes monitoring, including reporting of aggregate findings.
The Medical Director included participants according to their comprehensive history and physical examination, EKG, and blood analysis (metabolic and liver panels, hepatitis B, C and HIV screen, complete blood count, and thyroid panel). Further tests were done, including testosterone levels, when direct questioning revealed problems that warranted evaluation. Officers with debilitating symptoms had some priority; no preferential treatment was given for the number of meth-related exposures, age, gender, or police rank.
Patients included undercover, narcotics, and Special Weapons and Tactics (SWAT) officers from many Utah urban and county jurisdictions, Utah Highway Patrol (UHP), Immigration and Customs Enforcement (ICE), officers affiliated with the DEA, and officers exposed while performing chemical laboratory analyses.
The Intervention: The standard Hubbard sauna detoxification protocol. (Hubbard 1990)
OUTCOME EVALUATIONS
Symptom changes and quality of life were assessed using a baseline history and physical examination, follow-up interviews, and a series of pre- and post-treatment assessments:
- The RAND 36-item Short Form Health Survey (SF-36) assessed the 4-week health-related quality of life before treatment. The RAND SF-36 scoring mechanism differs from that licensed by Medical Outcomes Trust and produces a 9-scale profile of functional ability and physical and mental well-being. SF-36 scores were also compared pre- and post-treatment and to RAND US adult population norms.
- A 50-item pre- and post-treatment survey of the preceding 4 weeks’ symptoms, sick days, and sleep patterns was developed by the Foundation for Advancements in Science and Education (FASE) for clinical settings using the Hubbard regimen.
- A 13-item pre- and post-treatment neurotoxicity questionnaire based on the parameters of Singer (2006) rated the preceding 3 weeks’ problems involving irritability, social withdrawal, decreased motivation, recent memory, concentration, mental slowness/fog, sleep disturbances, fatigue, frequency and severity of headaches, sexual dysfunction, extremity numbness, and decreased mental sharpness, expressed on a 0–10 Likert-type scale.
- The Mini-Mental State Examination.
- Daily report form: a structured summary of vital signs/events recorded by trained staff of each treatment day, including any undesirable effects (whether or not related to treatment). For safety evaluation, any adverse events or interruptions of the protocol appear on the daily report form and are assessed by the Medical Director.
RESULTS
Treatment Length and Completion Rates
A total of 66 men and 3 women, averaging 44.6 years of age enrolled sequentially with a 92.8% completion rate; 5 men did not complete the treatment. Mean treatment length for the 64 patients who completed the treatment was 33 days.
Symptoms present in more than 50% of the officers recorded in the enrollment evaluation included fatigue: 96%, insomnia: 91%, headaches: 90%, heartburn: 81%, personality changes: 78%, numbness in hands and/or feet: 77%, memory loss: 77%, allergic history: 75%, poor concentration: 75%, back pain: 71%, joint pains: 71%, shortness of breath on exertion: 70%, skin irritation: 68%, anxiety/depression: 65%, abdominal gas/pain: 65%, sinusitis/congestion: 55%, and sore throat: 52%.
Percentage of officers with abnormal findings at enrollment: The abnormal findings included elevated blood lipids: 58%, elevated liver function tests: 41%, positive rombergism (inability to maintain balance in a “tandem stance” without visual input): 35%, hypertension: 28%, high blood glucose: 19%, low blood testosterone: 17%, and low blood thyroid: 17%.
Regimen Safety
Discomforts or other “adverse event” (which phrase means having emotional or illness-like symptoms) did not interfere significantly with the delivery of the program. For example every participant experienced the transitory flush or itchiness caused commonly by niacin but this did not interfere with delivery and participants completing the program. As Table 2 shows, many participants experienced temporary effects such as feeling despondent, having momentary cough, fatigue, etc. These were all transitory and did not require medical consultation. Sleeplessness did alter program delivery occasionally. After a night with less than full sleep the program was delivered in a reduced capacity the next day. Two officers had gout, one of which discontinued the program.
RAND SF-36 SCORES:
Change in Health-Related Quality of Life
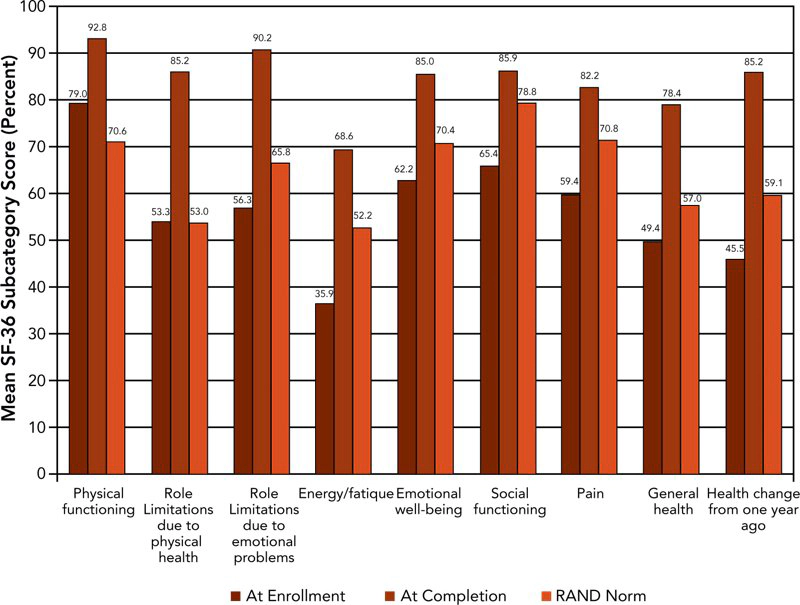
Figure 2 displays in graphic form the mean pre- and post-treatment SF-36 scores calculated using RAND methodology and compared with US population norms for those officers who completed the regimen.
Mean values of the officer pre-treatment health-related quality of life scores were significantly lower than RAND population norms in all nine sub-scales except role limitations due to physical health and role limitations due to emotional problems. Post-treatment, the officers’ scores showed statistically significant improvements when compared with pre-treatment scores. Officers’ post-treatment scores were also significantly improved for all sub-scales compared with RAND population norms.
Symptom Severity and Poor Health Days
Mean pre- and post-treatment symptom severity scores are shown in Figure 3 and are significantly reduced post-treatment versus pre-treatment.
Patients reported means of:
- 9.3 days of poor physical health pre-treatment, improving to 1.8 days by completion;
- 6.3 days of poor mental health pre-treatment versus 1.4 days by completion;
- 4.3 days of limited activities due to poor health pre-treatment versus 0.2 days by completion; and
- 2.0 sick days pre-treatment versus 0.3 days by completion.
Sleep Patterns
Participants averaged 5.8 hours of sleep per night pre-treatment, which improved to 7.6 hours on completion.
Neurotoxicity Scores
This questionnaire was administered from officer #20 onward. Excluding incomplete data, there were 38 matched pairs of pre- and post-treatment responses (84.4% response rate). The mean pre-treatment neurotoxicity score was 65.5, while the post-treatment mean score was 14.6.
Mini-Mental Status Evaluation
On a 30-point scale, scores below 25 indicate significant cognitive dysfunction. No measurable change was detected comparing mean pre- and post-treatment scores.
Discussion
Police officers generally require robust physical qualifications and emotional stability. In contrast to job selection criteria, the officers treated in this project had chronic debilitating symptoms consistent with chemical exposures.
In this small group of 69 individuals, it is surprising that 2 subsets of 17% of patients showed low thyroid and/or low testosterone status. Prevalence of hypothyroidism in the United States is about 5%. Preexisting thyroid imbalance may predispose officers to chronic illness, but low thyroid status may have directly resulted from methamphetamine-related exposures, in light of the causal relationship between environmental chemicals and low thyroid function.
Also unusual was the symptoms in common among those reporting chronic ill health. More than 75% of officers reported all the following nine symptoms: fatigue, insomnia, headaches, heartburn, personality changes, numbness in hands and/or feet, memory loss, a prior history of allergy symptoms, and poor concentration. This symptom cluster raises the possibility that “exposures in common” may have triggered “symptoms in common.” This symptom pattern may help future researchers or treatment professionals better recognize or classify methamphetamine-related exposures. The “pre-treatment” SF-36 scores of methamphetamine-exposed officers indicated more pain, more fatigue, and sign of significantly poorer health than the general population.
It is within this context that the Hubbard sauna-based treatment protocol was utilized. If chemical exposures and/or contamination caused these chronic symptoms, then a multifaceted “detoxification program” was a reasonable approach.
To our knowledge, this is the first time a sauna-based “detoxification program” has been evaluated in methamphetamine-exposed police officers. The vast majority completed the regimen with minimal discomfort or inconvenience, achieving significant reductions in their symptoms and measurably improved the health and quality of life. This suggests that this program could help similarly exposed police officers elsewhere.
| Number who experienced event | Number who missed days due to event | Number requesting medical consult due to event | Number who discontinued program due to event | ||||||
| Niacin flush, itchy skin | 69 | 0 | 0 | 0 | |||||
| Emotional, irritable, despondent | 18 | 0 | 0 | 0 | |||||
| Cough, congestion, sore throat | 13 | 0 | 0 | 0 | |||||
| Flu-like symptoms, no fever | 11 | 0 | 0 | 0 | |||||
| Flu-like symptoms with mild fever | 2 | 0 | 0 | 0 | |||||
| Headache | 6 | 0 | 0 | 0 | |||||
| Sleeplessness, vivid dreams | 15 | 12a | 0 | 1b | |||||
| Fatigue | 14 | 0 | 0 | 0 | |||||
| Stomach cramps, nausea, diarrhea | 8 | 3 | 0 | 0 | |||||
| Body aches | 5 | 2 | 0 | 0 | |||||
| Gout | 2c | 2 | 1 | 1 | |||||
| Work or other schedule conflicts | 5 | 4 | 0 | 3d | |||||
|
aPer protocol, patients who achieve less than 6.5 h of sleep have their next day’s treatment shortened to 10 min of exercise and 4 sauna sessions of 10 min each separated by 10-min breaks. bThis patient reported substantial health improvement but had insufficient sleep throughout the program. Treatment is considered incomplete for purposes of all data analysis. cBoth patients reported episodes of gout prior to starting the regimen. dTwo officers allotted insufficient treatment time and had to return to work; the third discontinued, citing work-related factors and also missed 6 days in the middle of the regimen. |
|||||||||
REFERENCES:
- Alexson O, Hogstedt C (1994) The health effects of solvents. In: Zenz C, Dickerson OB, and Horvath EP (eds) Occupational Medicine. St. Louis: Mosby Press, 764–768.
- Betsinger G (2006) Coping with meth lab hazards. Occupational Health and Safety 75(11): 50, 52, 54–58.
- Burgess JL (2001) Phosphine exposure from a methamphetamine laboratory investigation. Journal of Toxicology Clinical Toxicology 39(2): 165–168.
- Burgess JL, Barnhart S, and Checkoway H (1996) Investigating clandestine drug laboratories: adverse medical effects in law enforcement personnel. American Journal of Industrial Medicine 30(4): 488–494.
- Burgess JL, Kovalchick DF, Siegel EM, Hysong TA, and McCurdy SA (2002) Medical surveillance of clandestine drug laboratory investigators. Journal of Occupational and Environmental Medicine 44(2): 184–189.
- Carpenter DO, Arcaro K, and Spink DC (2002) Understanding the human health effects of chemical mixtures. Environmental Health Perspective 110(suppl 1): 25–42.
- CDC (2000) Public health consequences among first responders to emergency events associated with illicit methamphetamine laboratories—selected states, 1996–1999. MMWR Morbidity and Mortality Weekly Report 49(45): 1021–1024.
- CDC (2003) Recognition of illness associated with exposure to chemical agents—United States, 2003. MMWR Morbidity and Mortality Weekly Report 52(39): 938–940.
- CDC (2005) Acute public health consequences of methamphetamine laboratories—16 states, January 2000–June 2004. MMWR Morbidity and Mortality Weekly Report 54(14): 356–359.
- Cecchini M, LoPresti V (2007) Drug residues store in the body following cessation of use: impacts on neuroendocrine balance and behavior—use of the Hubbard sauna regimen to remove toxins and restore health. Medical Hypotheses 68(4): 868–879.
- Cecchini MA, Root DE, Rachunow JR, and Gelb PM (2006) Chemical exposures at the World Trade Center: use of the Hubbard sauna detoxification regimen to remove toxins and restore health. Townsend Letter 273: 58–65.
- Crinnion W (2007) Components of practical clinical detox programs—sauna as a therapeutic tool. Alternative Therapies in Health and Medicine 13(2): S154–S156.
- Dahlgren J, Cecchini M, Takhar H, and Paepke O (2007) Persistent organic pollutants in 9/11 World Trade Center rescue workers: reduction following detoxification. Chemosphere 69(8): 1320–1325.
- EHP Forum (1998) The threat of meth. Environmental Health Perspectives 106: A172–A173.
- Folstein MF, Folstein SE, and McHugh PR (1975) ‘‘Mini-mental state’’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12(3): 189–198.
- Garwood ER, Bekele W, McCulloch CE, and Christine CW (2006) Amphetamine exposure is elevated in Parkinson’s disease. Neurotoxicology 27(6): 1003–1006.
- Hall HV, McPherson SB, Twemlow SW, and Yudko E (2003) Epidemiology. In: Yudko E, Hall HV, and McPherson SB (eds) Methamphetamine Use: Clinical and Forensic Aspects. Boca Raton: CRC Press, 13–15.
- Hays RD, Sherbourne CD, and Mazel RM (1993) The RAND 36-Item Health Survey 1.0. Health Economics 2(3): 217–227.
- Herpin G, Gargouri I, Gauchard GC, Nisse C, and Khadhraoui M, Elleuch B, et al. (2009) Effect of chronic and subchronic organic solvents exposure on balance control of workers in plant manufacturing adhesive materials. Neurotoxicity Research 15(2): 179–186.
- Hollowell JG, Staehling NW, and Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of Clinical Endocrinology and Metabolism 87(2): 489–499.
- Hubbard LR (1990) Clear Body, Clear Mind. 2002 ed. Los Angeles: Bridge Publications.
- Kilburn KH, Warsaw RH, and Shields MG (1989) Neurobehavioral dysfunction in firemen exposed to polychlorinated biphenyls (PCBs): possible improvement after detoxification. Archives of Environmental Health 44(6): 345–350.
- Leonard KL. (2008). Is patient satisfaction sensitive to changes in the quality of care? An exploitation of the Hawthorne effect. Journal of Health Economics 27(2): 444–59.
- Levisky JA, Bowerman DL, Jenkins WW, Johnson DG, and Karch SB (2001) Drugs in postmortem adipose tissues: evidence of antemortem deposition. Forensic Science International 121(3): 157–160.
- Marshall DR (2000) Report before the 106th congress: emerging drug threats and perils facing Utah’s youth. Salt Lake City, UT: Committee on the Judiciary, United States Senate. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname. 106_senate_ hearings&docid. f:73821.pdf (accessed 17 April 2011)
- Martyny JW, Arbuckle SL, McCammon CS, Esswein EJ, and Erb N (2004) Chemical exposures associated with clandestine methamphetamine laboratories. Denver, CO: National Jewish Medical and Research Center www.nationaljewish.org/pdf/chemical_ exposures.pdf. (accessed 17 April 2011).
- Martyny JW, Van Dyke MV, McCammon CS, Erb N, and Arbuckle SL (2005a) Chemical exposures associated with clandestine methamphetamine laboratories using the anhydrous ammonia method of production. Denver, CO: National Jewish Medical and Research Center. http://www.njc.org/pdf/Ammonia%20Meth.pdf. (accessed 17 April 2011).
- Martyny JW, Van Dyke M, McCammon CS, Erb N, Arbuckle SL (2005b) Chemical exposures associated with clandestine methamphetamine laboratories using the hypophosphorous and phosphorous flake method of production. National Jewish Medical Research Center http://www.njc.org/pdf/meth-hypo-cook.pdf (Accessed 9 Feb 2011).
- Miller MD, Crofton KM, Rice DC, and Zoeller RT (2009) Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environmental Health Perspectives 117(7): 1033–1041.
- Rea WJ, Pan Y, Johnson AR, Ross GH, Suyama H, and Fenyves EJ (1996) Reduction of chemical sensitivity by means of heat depuration, physical therapy and nutritional supplementation. Journal of Nutritional and Environmental Medicine 6: 141–148.
- Schep LJ, Slaughter RJ, and Beasley DM (2010) The clinical toxicology of metamfetamine. Clinical Toxicology (Philadelphia) 48(7): 675–694.
- Schnare DW, Ben M, and Shields MG (1984) Body burden reduction of PCBs, PBBs and chlorinated pesticides in human subjects. Ambio 13: 378–380.
- Schnare DW, Denk G, Shields M, and Brunton S (1982) Evaluation of a detoxification regimen for fat stored xenobiotics. Medical Hypotheses 9(3): 265–282.
- Sharpe RM (2003) The “oestrogen hypothesis”—where do we stand now? International Journal of Andrology 26(1): 2–15.
- Singer R (2006) Neurotoxicity Guidebook. San Diego, CA: Aventine Press, 3.
- Witter RZ, Martyny JW, Mueller K, Gottschall B, and Newman LS (2007) Symptoms experienced by law enforcement personnel during methamphetamine lab investigations. Journal of Occupational and Environmental Hygiene 4(12): 895–902.
- Thrasher DL, Von Derau K, and Burgess J (2009) Health effects from reported exposure to methamphetamine labs: a poison center-based study. Journal of Medical Toxicology 5(4): 200–204.
- Tretjak Z, Beckmann S, Tretjak A, and Gunnerson C (1989) Report on occupational, environmental, and public health in Semic: a case study of polychlorinated biphenyl (PCB) pollution. In: Post-Audits of Environmental Programs and Projects; Proceedings, Environmental Impact Analysis Research Council / ASCE. New Orleans, LA, 57–72.
- Tretjak Z, Shields M, and Beckmann SL (1990) PCB reduction and clinical improvement by detoxification: an unexploited approach? Human and Experimental Toxicology 9(4): 235–244.
- Tsyb AF, Parshkov EM, Barnes J, Yarzutkin VV, Vorontsov NV, and Dedov VI (1998) Proceedings of the 1998 International Radiological Post Emergency Response Issues Conference. Washington, DC: US EPA, 162–166, efile pages 178–182. Witter RZ, Martyny JW, Mueller K, Gottschall B, and Newman LS (2007) Symptoms experienced by law enforcement personnel during methamphetamine lab investigations. Journal of Occupational and Environmental Hygiene 4(12): 895–902.
- Woodruff TJ (2011) Bridging epidemiology and model organisms to increase understanding of endocrine disrupting chemicals and human health effects. The Journal of Steroid Biochemistry and Molecular Biology 127(1–2): 108–117.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. (2010) Identification of late-onset hypogonadism in middle-aged and elderly men. The New England Journal of Medicine 363(2): 123–135.

